The world's first verification of agreement of the condensing heat-transfer to hydrogen with the Nusselt theory
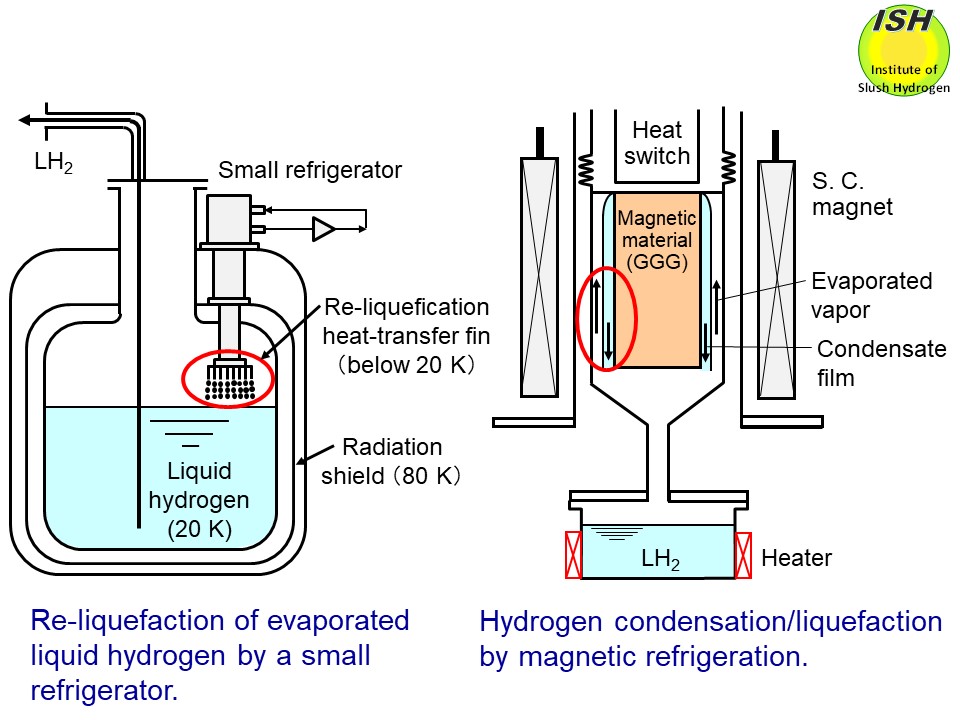
In the condensation and liquefaction of hydrogen, the re-liquefaction
of evaporated gas in the liquid hydrogen tank for the high-temperature
SMES (Superconducting Magnetic Energy Storage) is assumed as illustrated
in the above figure for the high-efficiency hydrogen energy system. In the case of liquid
helium, certain types of medical-use MRI (Magnetic Resonance Imaging) equipment
are already provided with the small refrigerator for re-liquefaction. Nevertheless,
there has been very little experimental work on the condensing heat-transfer
to cryogenic fluids*.
These re-liquefaction systems require high performance condensing heat
exchangers, which are to have the high heat flux and small heat-transfer
temperature difference across the condensate film. However, only few reliable
experimental data are available on the condensing heat-transfer to cryogenic
fluids such as hydrogen, deuterium, and helium [31].
In the world’s first demonstration of the hydrogen liquefaction experiment
undertaken by means of the magnetic refrigeration (cf. Magnetic refrigeration)
[8, 9], it was necessary to design a experimental apparatus using a thermo-syphon
type high-performance heat pipe operated at the temperature difference
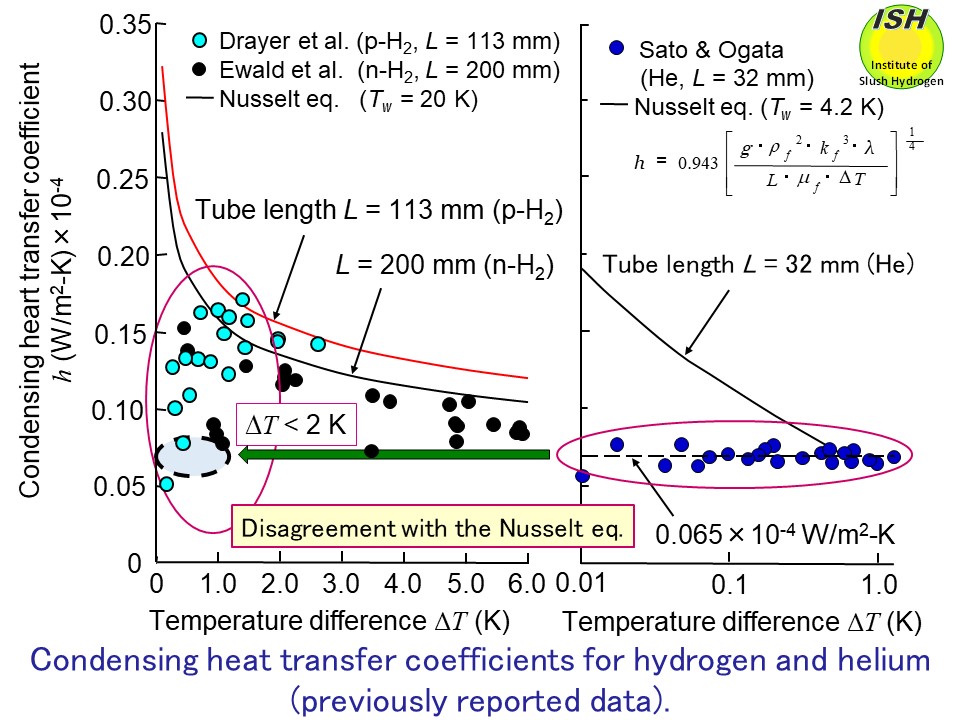
∆T of within 2 K (ΔT < 2 K), as illustrated in the above figure. To clarify the relationship between the condensing heat-transfer coefficient
measured in previously reported experiments and the theoretical Nusselt
eq. [31], we did further experiments on the condensation of hydrogen and
nitrogen, which is also important to develop a high-performance and compact
heat exchanger (condenser) for liquefaction or re-liquefaction of hydrogen.
The process of film-condensation on a vertical wall is illustrated in
the above figure. The well-known theoretical analysis of laminar film-condensation on the
vertical wall was made by W. Nusselt. The experimental investigations with
nitrogen and oxygen have shown a good agreement, within experimental error,
between experimental data and those predicted by the Nusselt eq. On the
other hand, in the investigations with cryogenic fluids such as hydrogen,
deuterium, and helium, the experimental condensing heat-transfer coefficients
have fallen below those predicted by the Nusselt eq., and diverged with
decreasing temperature difference ∆T across the condensate film, as shown in the figure below.
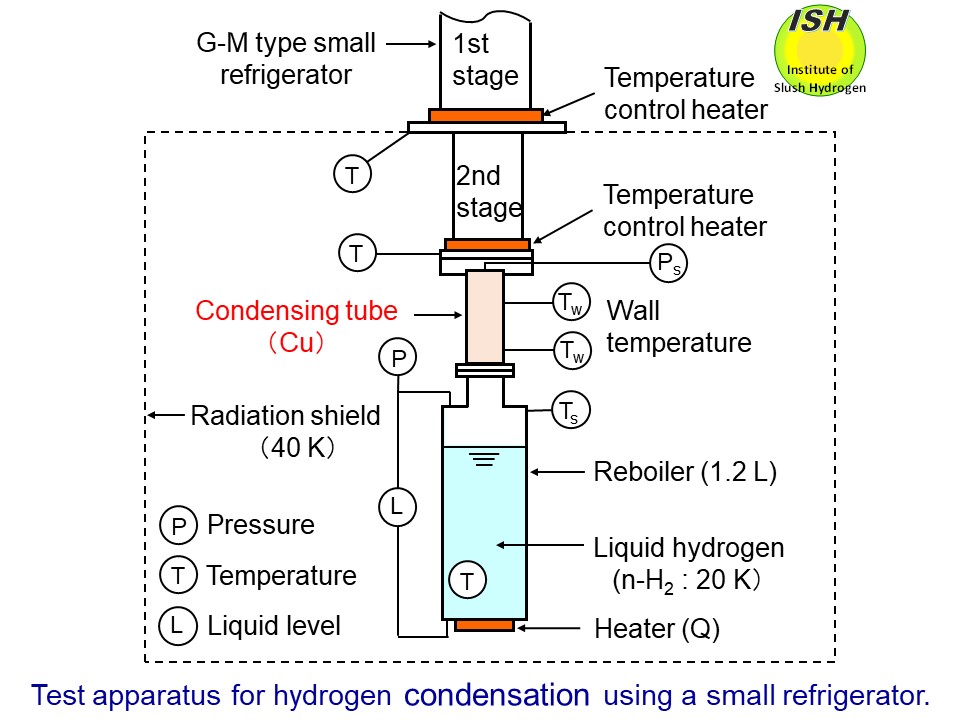
The test apparatus using a G-M type small refrigerator (UCR31W made by
MHI) to investigate the condensing heat-transfer for hydrogen and nitrogen
is illustrated in the figure below. The hydrogen vapor, which is generated by continuously vaporizing liquid
hydrogen in the reboiler by means of an electrical heater, flows up into
the condensing tube, while the liquid condensate flows down the tube in
annular film along the tube wall. Our experimental condensing heat-transfer
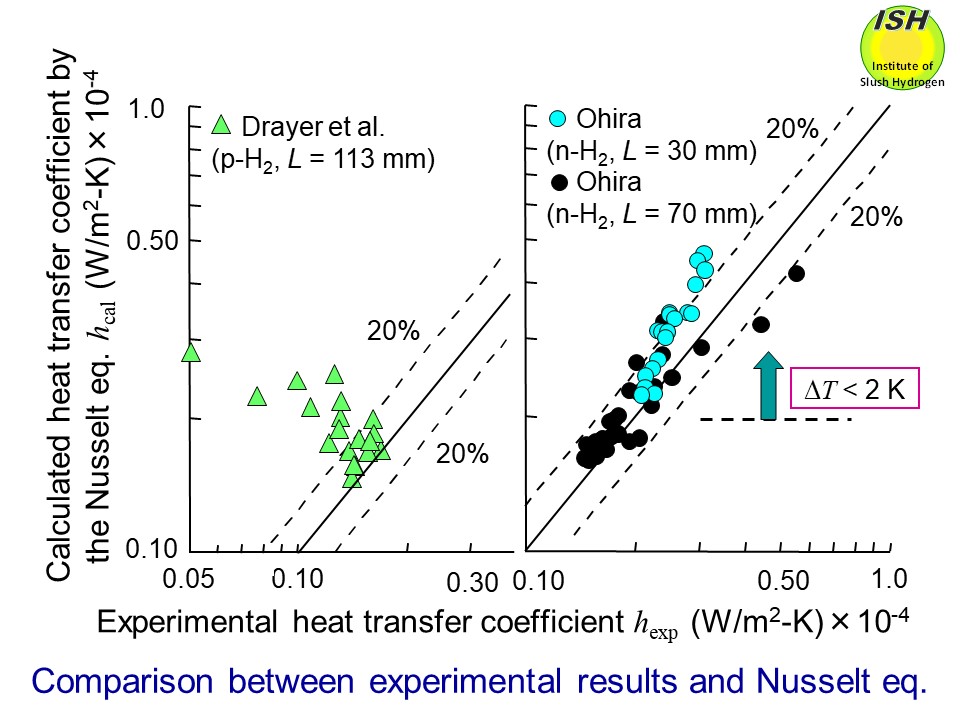
coefficients for hydrogen and the previously reported data by Drayer et
al. are compared with the Nusselt eq. as shown in the figure below. We have firstly verified that the condensing heat-transfer coefficients
for hydrogen obtained from the present experiment are in good agreement
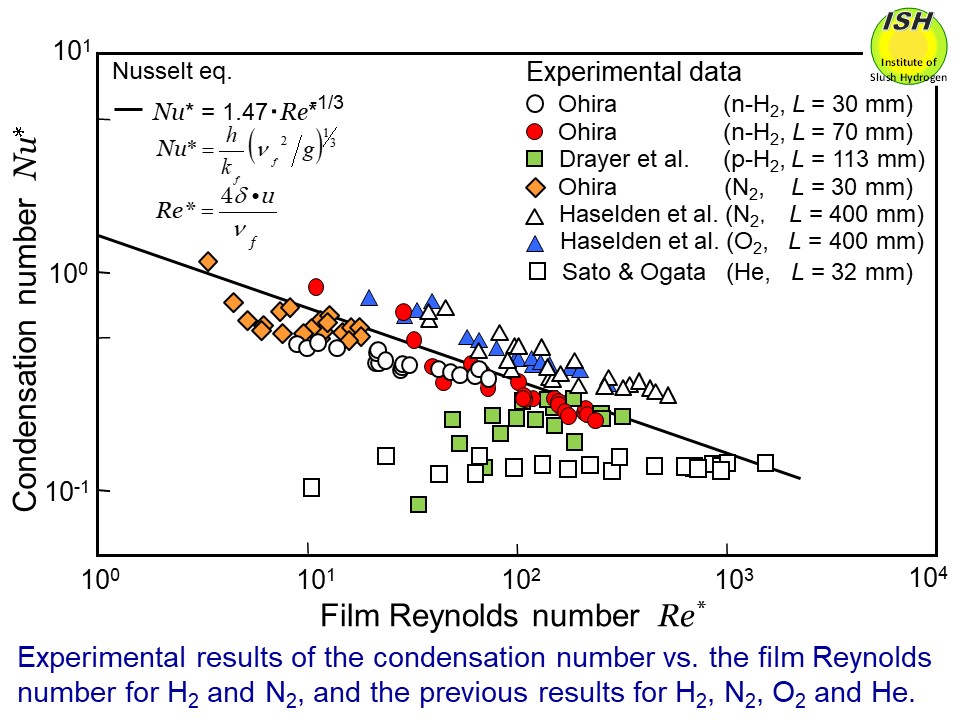
with the Nusselt eq. within about ±20%. Relationships between the condensation number Nu* and the film Reynolds number Re* for nitrogen and hydrogen, as obtained from the present experiment are
shown in the figure below. Also, the previously reported experimental data for oxygen, nitrogen,
hydrogen and helium, and calculation results (solid line) derived from
the Nusselt eq. are shown for comparison [6, 7].
The present study obtained in the experiments suggests that the condensing
heat-transfer coefficients for deuterium and helium might also be obtained
using the Nusselt eq. when their condensate liquid films are in laminar
flow. This result is important in thermal and cryogenic engineerings such as
the re-liquefaction of evaporated gas in the liquid hydrogen tank for the
high-temperature SMES etc. and in the liquid helium reservoir for MRI equipments.
* Cryogenic fluid: The normal boiling point temperature is below -150℃
(123 K) or 100 K (-173℃), such as methane, oxygen, nitrogen, neon, hydrogen,
and helium.
Comparison between the previous and the present author's data